Utility functions and commonly used science property calculators. More...
Go to the source code of this file.
Macros | |
| #define | UNIV_GAS_CONST_ 8.314472 |
Functions | |
| static double | mean_speed__m_s (double temperature__K, double mw__kg_mol) |
| static double | mean_free_path__m (double diffusion_coeff__m2_s, double temperature__K, double mw__kg_mol) |
| static double | transition_regime_correction_factor (double mean_free_path__m, double radius__m, double alpha) |
| static double | d_transition_regime_correction_factor_d_radius (double mean_free_path__m, double radius__m, double alpha) |
| static double | gas_aerosol_transition_rxn_rate_constant (double diffusion_coeff__m2_s, double mean_free_path__m, double radius__m, double alpha) |
| static double | d_gas_aerosol_transition_rxn_rate_constant_d_radius (double diffusion_coeff__m2_s, double mean_free_path__m, double radius__m, double alpha) |
| static double | gas_aerosol_continuum_rxn_rate_constant (double diffusion_coeff__m2_s, double mean_speed__m_s, double radius__m, double alpha) |
| static double | d_gas_aerosol_continuum_rxn_rate_constant_d_radius (double diffusion_coeff__m2_s, double mean_speed__m_s, double radius__m, double alpha) |
Detailed Description
Utility functions and commonly used science property calculators.
Definition in file util.h.
Macro Definition Documentation
◆ UNIV_GAS_CONST_
Function Documentation
◆ d_gas_aerosol_continuum_rxn_rate_constant_d_radius()
|
inlinestatic |
Calculate the derivative of the continuum-regime gas-aerosol reaction rate constant by particle radius [Tie2003]
\[ \frac{dk_c}{dr} = 4 \pi \frac{\frac{r^2}{D_g} + \frac{8r}{v(T) \gamma}}{\left(\frac{r}{D_g} + \frac{4}{v(T) \gamma}\right)^2} \]
where \(r\) is the particle radius [m], \(D_g\) is the gas-phase diffusion coefficient of the reactant [ \(\mbox{m}^2\mbox{s}^{-1}\)], \(\gamma\) is the reaction probability [unitless], and v is the mean free speed of the gas-phase reactant.
- Parameters
-
diffusion_coeff__m2_s Diffusion coefficient of the gas species [ \(\mbox{m}^2\, \mbox{s}^{-1}\)] mean_speed__m_s Mean speed of the gas molecule [m s-1] radius__m Particle radius [m] alpha Mass accomodation coefficient [unitless]
Definition at line 209 of file util.h.


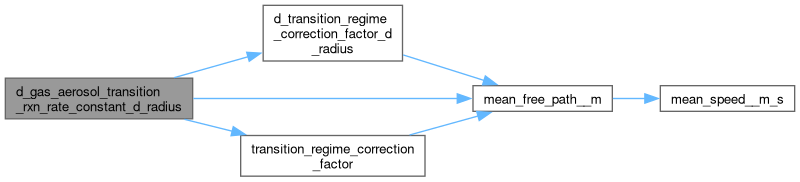
◆ d_gas_aerosol_transition_rxn_rate_constant_d_radius()
|
inlinestatic |
Calculate the derivative of a transition-regime gas-aerosol reaction rate by particle radius
\[ \frac{d k_c}{d r} = 4 \pi D_g ( f_{fs} + r \frac{d_{fs}}{d r} ) \]
where \(r\) is the radius of the particle(s) [m], \(D_g\) is the diffusion coefficient of the gas-phase species [ \(\mbox{m}^2\, \mbox{s}^{-1}\)] and \(f_{fs}( K_n, \alpha )\) is the Fuchs Sutugin transition regime correction factor [unitless] ( \(K_n\) is the Knudsen Number [unitess] and \(\alpha\) is the mass accomodation coefficient.
- Parameters
-
diffusion_coeff__m2_s Diffusion coefficent of the gas species [ \(\mbox{m}^2\, \mbox{s}^{-1}\)] mean_free_path__m Mean free path of gas molecules [m] radius__m Particle radius [m] alpha Mass accomodation coefficient [unitless]
Definition at line 154 of file util.h.
◆ d_transition_regime_correction_factor_d_radius()
|
inlinestatic |
Calculate the derivative of the transition regime correction factor by particle radius.
\[ \frac{d f_{fs}}{d r} = \frac{0.75 \alpha \lambda (K_n^2 + 2K_n + 1 + (0.283-0.75)\alpha)}{r^2(K_n^2 + (1+0.283\alpha)K_n + 0.75\alpha)^2} \]
- Todo
- double check the correction factor derivative equation where the Knudsen Number \(K_n = \lambda / r\) (where \(\lambda\) is the mean free path [m] of the gas-phase species and \(r\) is the effective radius of the particles [m]), and \( \alpha \) is the mass accomodation coefficient, which is typically assumed to equal 0.1 [Zaveri2008].
- Parameters
-
mean_free_path__m mean free path of the gas-phase species [m] radius__m Particle effective radius [m] alpha Mass accomodation coefficient [unitless]
Definition at line 91 of file util.h.


◆ gas_aerosol_continuum_rxn_rate_constant()
|
inlinestatic |
Calculate the gas-aerosol reaction rate for the continuum regime [Tie2003] [ \(\mbox{m}^3\, \mbox{particle}^{-1}\, \mbox{s}^{-1}\)]
The rate constant \(k_c\) is calculated as:
\[ k_c = \frac{4 \pi r^2_e}{\left(\frac{r}{D_g} + \frac{4}{v(T)\gamma}\right)} \]
where \(r\) is the particle radius [m], \(D_g\) is the gas-phase diffusion coefficient of the reactant [ \(\mbox{m}^2\mbox{s}^{-1}\)], \(\gamma\) is the reaction probability [unitless], and v is the mean free speed of the gas-phase reactant.
- Parameters
-
diffusion_coeff__m2_s Diffusion coefficient of the gas species [ \(\mbox{m}^2\, \mbox{s}^{-1}\)] mean_speed__m_s Mean speed of the gas molecule [m s-1] radius__m Particle radius [m] alpha Mass accomodation coefficient [unitless]
Definition at line 183 of file util.h.
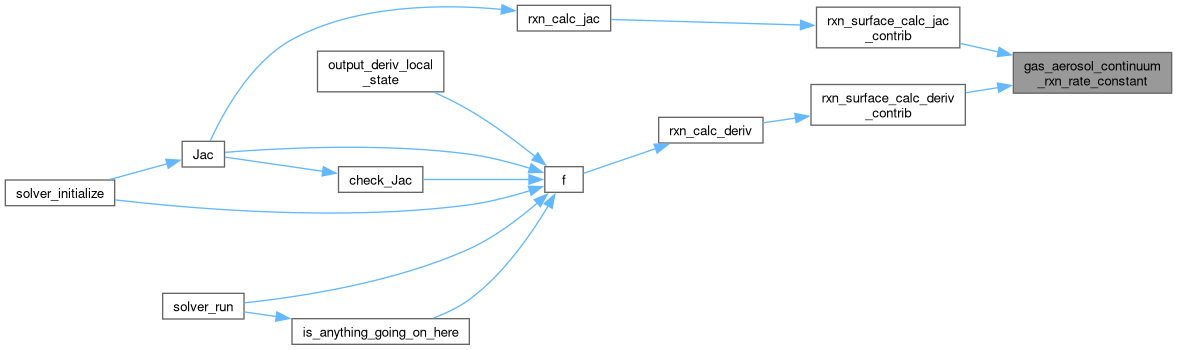
◆ gas_aerosol_transition_rxn_rate_constant()
|
inlinestatic |
Calculate the gas-aerosol reaction rate constant for the transition regime [ \(\mbox{m}^3\, \mbox{particle}^{-1}\, \mbox{s}^{-1}\)]
The rate constant \(k_c\) is calculated according to [Zaveri2008] as:
\[ k_c = 4 \pi r D_g f_{fs}( K_n, \alpha ) \]
where \(r\) is the radius of the particle(s) [m], \(D_g\) is the diffusion coefficient of the gas-phase species [ \(\mbox{m}^2\,\mbox{s}^{-1}\)], \(f_{fs}( K_n, \alpha )\) is the Fuchs Sutugin transition regime correction factor [unitless], \(K_n\) is the Knudsen Number [unitess], and \(\alpha\) is the mass accomodation coefficient.
Rates can be calculated as:
\[ r_c = [G] N_a k_c \]
where \([G]\) is the gas-phase species concentration [ppm], \(N_a\) is the number concentration of particles [ \(\mbox{particle}\,\mbox{m}^{-3}\)] and the rate \(r_c\) is in [ \(\mbox{ppm}\,\mbox{s}^{-1}\)].
- Parameters
-
diffusion_coeff__m2_s Diffusion coefficent of the gas species [ \(\mbox{m}^2\, \mbox{s}^{-1}\)] mean_free_path__m Mean free path of gas molecules [m] radius__m Particle radius [m] alpha Mass accomodation coefficient [unitless]
Definition at line 129 of file util.h.
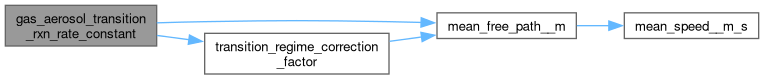
◆ mean_free_path__m()
|
inlinestatic |
Calculate the mean free path of a gas-phase species [m]
\[ \lambda = 3.0 D_g / v \]
where \(D_g\) is the gas-phase diffusion coefficient [ \(\mbox{m}^2\,\mbox{s}^{-1}\)] and \(v\) is the mean speed of the gas-phase molecules [ \(\mbox{m}\,\mbox{s}^{-1}\) ].
- Parameters
-
diffusion_coeff__m2_s Diffusion coefficient of the gas species [ \(\mbox{m}^2\, \mbox{s}^{-1}\)] temperature__K Temperature [K] mw__kg_mol Molecular weight of the gas-phase species [ \(\mbox{kg}\, \mbox{mol}^{-1}\)]
Definition at line 50 of file util.h.

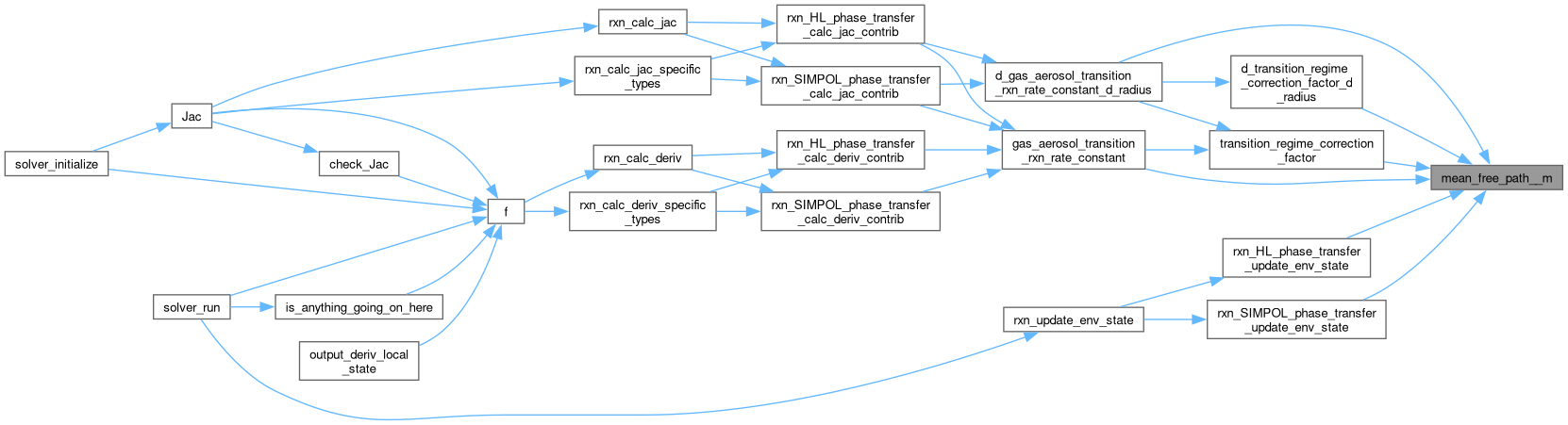
◆ mean_speed__m_s()
|
inlinestatic |
Calculate the mean speed of a gas-phase species [ \(\mbox{m}\,\mbox{s}^{-1}\) ] :
\[ v = \sqrt{\frac{8RT}{\pi MW}} \]
where R is the ideal gas constant [ \(\mbox{J}\, \mbox{K}^{-1}\, \mbox{mol}^{-1}\)], T is temperature [K], and MW is the molecular weight of the gas-phase species [ \(\mbox{kg}\, \mbox{mol}^{-1}\)]
- Parameters
-
temperature__K Temperature [K] mw__kg_mol Molecular weight of the gas-phase species [ \(\mbox{kg}\, \mbox{mol}^{-1}\)]
Definition at line 30 of file util.h.
◆ transition_regime_correction_factor()
|
inlinestatic |
Calculate the transition regime correction factor [unitless] [Fuchs1971] :
\[ f(K_n,\alpha) = \frac{0.75 \alpha ( 1 + K_n )}{K_n(1+K_n) + 0.283\alpha K_n + 0.75 \alpha} \]
where the Knudsen Number \(K_n = \lambda / r\) (where \(\lambda\) is the mean free path [m] of the gas-phase species and \(r\) is the effective radius of the particles [m]), and \( \alpha \) is the mass accomodation coefficient, which is typically assumed to equal 0.1 [Zaveri2008].
- Parameters
-
mean_free_path__m mean free path of the gas-phase species [m] radius__m Particle effective radius [m] alpha Mass accomodation coefficient [unitless]
Definition at line 69 of file util.h.

CAMP 1.0.0 documentation generated by doxygen 1.12.0